From “100% accurate” to no better than a coin toss, the reputation of COVID-19 serology testing in media coverage has been on a rollercoaster ride in recent months.
Since the start of January 2020, media mentions of antibody testing went up, then down, then up again; the test was described as great, then it was bad, then it was good again…
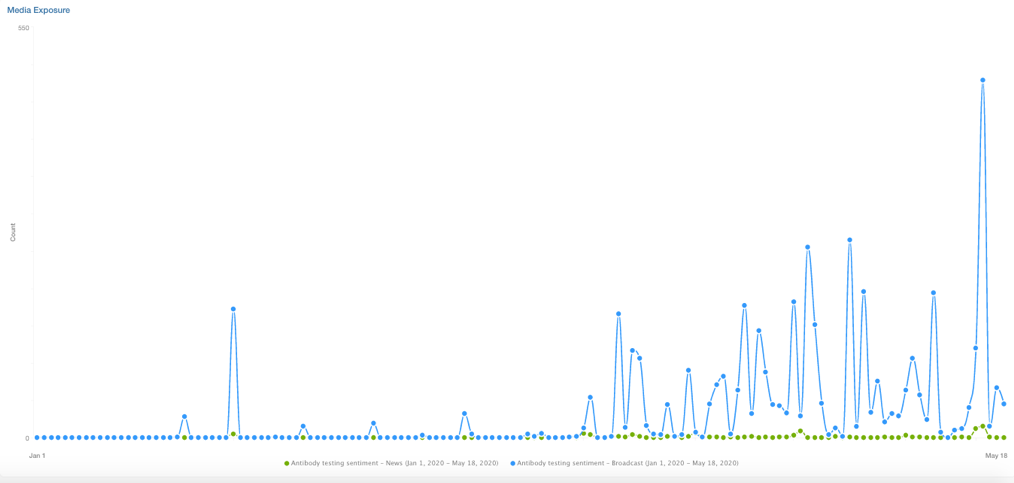
Some hope that an antibody test could reveal previous infections and pave the way to return to pre-virus liberties. Epidemiologists issue words of caution. Media sentiment of antibody tests has lurched between wild enthusiasm and damning.
If your business is launching an antibody test soon, you will have to weather weeks of erratic media reporting on antibody tests.
Therefore, providing strong and clear evidence of the effectiveness of your test is more important now than previously. Taking extra time to brief media about your product will ultimately help them tell your story. Journalists are time-poor. Briefing them with visual materials (imagery, videos, animations) or documentation using simple terminology will benefit you. It is important to select media outlets who have accurately reported antibody testing previously and reporters or journalists who have a good understanding of science and health. We have put together a downloadable checklist to assist you.
London Agency’s team have biomedical science degrees and some of us have even worked in pathology labs in Australia and overseas, but even we are finding recent media coverage perplexing. So, if you are puzzled, you are not alone!
In the early stages of the COVID-19 outbreak, the media portrayed antibody testing positively. It was seen as a promising method to diagnose the virus. Serology testing is a reliable diagnostic tool to diagnose HIV and other pathogens, so perhaps it could also for COVID-19. Some countries went ahead and bulk bought the kits to be sold as home-testing solutions to control the spread of the virus.
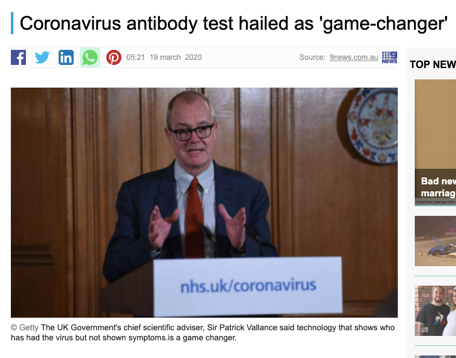
However, the scientific community quickly warned governments against antibody testing for COVID-19 diagnosis. Widespread antibody testing could help us better understand the immune response to the virus. However, this technique is not suited to diagnosing infections in the middle of a pandemic.
COVID-19 antibodies develop several days after infection, so patients tested too early would receive a negative result that may give them false reassurance about their status. The Royal College of Pathologists of Australasia released a position statement advising against using serology testing for the detection of COVID-19 in the early stages of the virus.
Media quickly picked up on scientific community concerns and began warning people against antibody testing. News that test kits shipped from China were faulty further damaged the reputation of serology testing.
The Doherty Institute in Melbourne conducted a post-market validation study of three Therapeutic Goods Administration-approved different serological assays. They found that one of the tests’ specificity and sensitivity results were in line with the manufacturer’s performance criteria. However, the conclusion was that serological assays have no role in the diagnosis of acute COVID-19 infection.
In particular, this brutal article published in Fairfax online media last week. It stated that manufacturers with no experience in selling medical or diagnostic equipment now have TGA-approved antibody tests. No diagnostics company wants to find themselves in an article like this one.
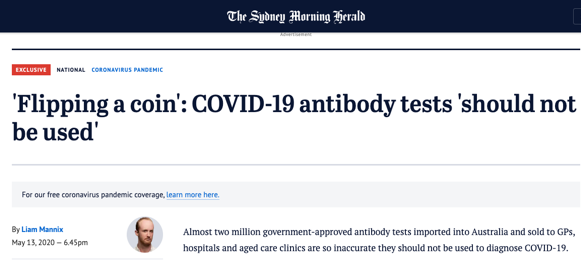
Despite the varying specificity and sensitivity of COVID-19 serology tests, the media rarely distinguishes one manufacturer from another. Some media outlets acknowledge that not all are the same, whilst others suggest that even the most accurate serology tests have no place in the diagnosis of COVID-19. We might accidentally misinterpret negative or positive media coverage as representative of all tests, as opposed to an individual manufacturer’s test.
On 14th May, the UK announced the widespread use of Roche’s antibody test. The media widely dubbed it as “100 percent accurate”. An amazing headline, but probably likely to cause perspiration among the company’s Scientific Director and Public Affairs Director. Few things in science are ever that accurate. If you are 100 percent accurate, then the only way is down. A great day of media coverage now represents a risk to the company’s reputation if there is ever an issue with future tests.
UK immunologist Dr Michael Browning wrote in the Guardian of his concerns around antibody tests:
‘While the availability of antibody testing undoubtedly represents an important step in the fight against Covid-19, limitations mean that these tests are not as clinically useful as the public has been led to believe’.
Coronavirus testing is a fast-moving, complicated, and unfamiliar topic. After all, pathology testing does not usually get this much media coverage.
Journalists are doing their best to keep up with daily COVID-19 updates, however there is still miscommunication. This is partly because the public wants to hear good news. We hope that testing that will help us return to our ‘old lives’. And preferably we want it yesterday!
A downloadable checklist for pitching stories about diagnostic tests in COVID-19 is available here to help avoid media muddles.