
When COVID-19 reached Australia, no clinical trials were allowed to begin, and ongoing trials stopped recruiting. The safety of patients was prioritised, as we did not know much about the virus and the risk it posed to society. Current trials had to quickly shift to using remote systems.
After some time, trials began recruiting again and were able to start as normal with solutions in place to ensure the safety of those involved in the research.
Watch the Continuity of Care Collaboration webinar about clinical trials.
COVID-19 accelerated the uptake of tele-trials in Australia, supported by the availability of remote monitoring, telehealth and medicines delivery.
Tele-trials were initially conceived by the Clinical Oncology Society of Australia’s (COSA) Rural and Regional Group before the pandemic, to improve the access of remote and rural communities to clinical trials for cancer treatments.
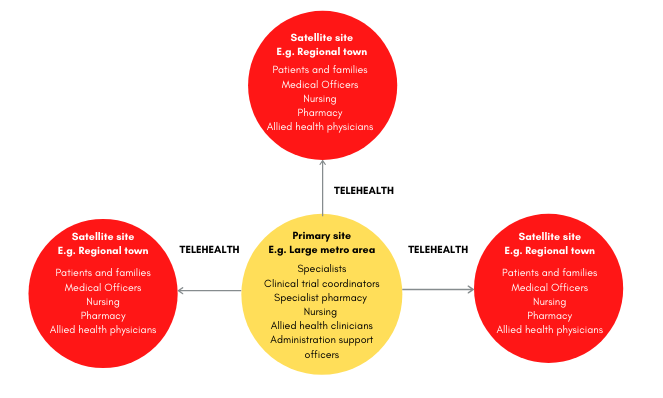
Trials are conducted within ‘clusters’, where primary sites and satellite sites work together using telehealth.
Medicines Australia works across industry and with Federal and State Governments to strengthen the policy and regulatory environment that will encourage more clinical trials, and increasingly the take-up of tele-trials, to come to Australia. There is big potential to take advantage of the clinical trial sector in Australia for tele-trials. In 2019 alone, there were 1,820 ongoing trials in Australia, a 22% increase on 2015. This contributes an estimated $1.1 billion a year to the economy.
The organisation, which represents the innovative medicines industry, is encouraging healthcare companies to embrace tele-trials in future set-ups: “We believe it’s no longer a matter of ‘Is it possible?’. It’s more about ‘How is it possible?’”
“We are currently working with state governments to finalise standard contract templates to allow healthcare companies to easily set up a tele-trial moving forward,” reinforces the organisation.
Some Phase I trials require specialised equipment and intense monitoring of participants, so may not be suited to the tele-trial model.
Management and transport of the medicinal product at satellite sites is an important consideration. Some trials use unregistered medication, so it is essential to know how that compound or medication was stored and travelled (e.g., temperature).
Zoe Armstrong, Clinical Research Director at MSD, said: “It’s complicated with unregistered medication. There is still a little bit of work to do on how to move unregistered medication around. Couriers need to know what they are shipping, so we need to tell them that the delivery is blinded! Open-label clinical trials are going to be able to transition to the tele-trial model more quickly.”
“Training pharmacists in remote areas on putting together a compounded medication can also present difficulties if they have not done this before.”
“Another important consideration is potential adverse events. We need to make sure trial participants know to report any unexpected affect. Therefore, more education is required.”
“Preserving the integrity of the trial is just as important as treating the patient.”
Medicines Australia Manager of Industry and Regulatory Policy, Peter Komocki, believes the transition to tele-trials in Australia needs time and consideration: “One of the challenges we anticipate is the cultural and mindset shift, including of patients and healthcare practitioners; together with a lack of practice and lack of awareness, infrastructure also needs to be managed carefully; getting the product to the patient rather than getting the patient to come to the product, and making sure the costs don’t become excessive to complete the trial.”
The recent Federal Budget announced $75.2 million to the Department of Health in Queensland for the Australian Tele-trial Program. A further $30.5 million was allocated to the NSW Ministry of Health on a complementary project increasing the opportunity for national consistency. These exciting announcements mean more patients in regional locations will be able to benefit from new, and possibly lifesaving, medicines.
Currently, less than 5% of regional cancer patients participate in any clinical trial according to an article featuring researchers from the Victorian Comprehensive Cancer Centre. With better understanding of how tele-trials can work effectively and with more funding, this number could increase in the years to come.
Medicines Australia firmly believes “tele-trials should be seen as an opportunity to improve regional healthcare team experience while also improving patient’s access to treatments they would otherwise not be able to receive. Increased government investment into regional areas and healthcare infrastructure also has the potential to create jobs.”
Additionally, the drop-out rate is higher for trial participants who are based more remotely. Zoe Armstrong said: “We want to do whatever we can to help patients stay in the trial. One of the difficulties in Australia is that we are not always successful with recruitment. Tele-trials give Australian patients the ability to access clinical trials in a choice of environments that may better fit their needs and assist them to remain in a clinical trial.”
“General feedback on our tele-trials so far is that they are incredibly impactful on the patient and the sites we work with.”
Peter Komocki adds: “Australia is one of the best places in the world for clinical trials, and we hope the best in the world for tele-trials. We have an excellent infrastructure and healthcare system, excellent research and diverse populations. And now with tele-trials, there is an added competitive advantage, that also allows more patients to benefit from cutting edge research and development.”
There are already patients participating in tele-trials across Australia (Queensland, New South Wales, Australian Capital Territory, Victoria and South Australia).
More information available on Medicines Australia website and on the COSA website.
Read our article about how COVID-19 impacted healthcare delivery in remote areas.