
On 1st January 2022, a revamped Therapeutic Goods Administration (TGA) Advertising Code came into effect, including several changes that will shake up the way the health industry markets to consumers.
Ambiguity has long been a word associated with the TGA Code. The changes seek to address these concerns. However, obscurity will remain beyond this reset and London Agency will endeavour to clarify those niggling grey areas. Look out for more content on this topic in the coming weeks and please do contact us if you have questions after reading this article.
It’s important to note that advertisers have until 30th June 2022 to transition to the new TGA Code.
Below we outline some key changes that medical companies should price into their marketing and PR strategies.
Paid or incentivised influencer testimonials banned
In what is the most controversial change, splashing across lifestyle columns of major news outlets in recent weeks, influencers will now be prohibited from paid or incentivised promotions of therapeutic goods.
The new TGA Code states:
The Code is unchanged for PR
This is unchanged from the 2018 iteration of the TGA Code. First things first, if you value media as a platform, you can keep doing what you’re doing because the TGA Code does not apply to “genuine news” that is published or broadcast in any medium by:
However, when it comes to determining what is “genuine news”, London Agency advises seeking out the opinion of a professional with news or PR experience.
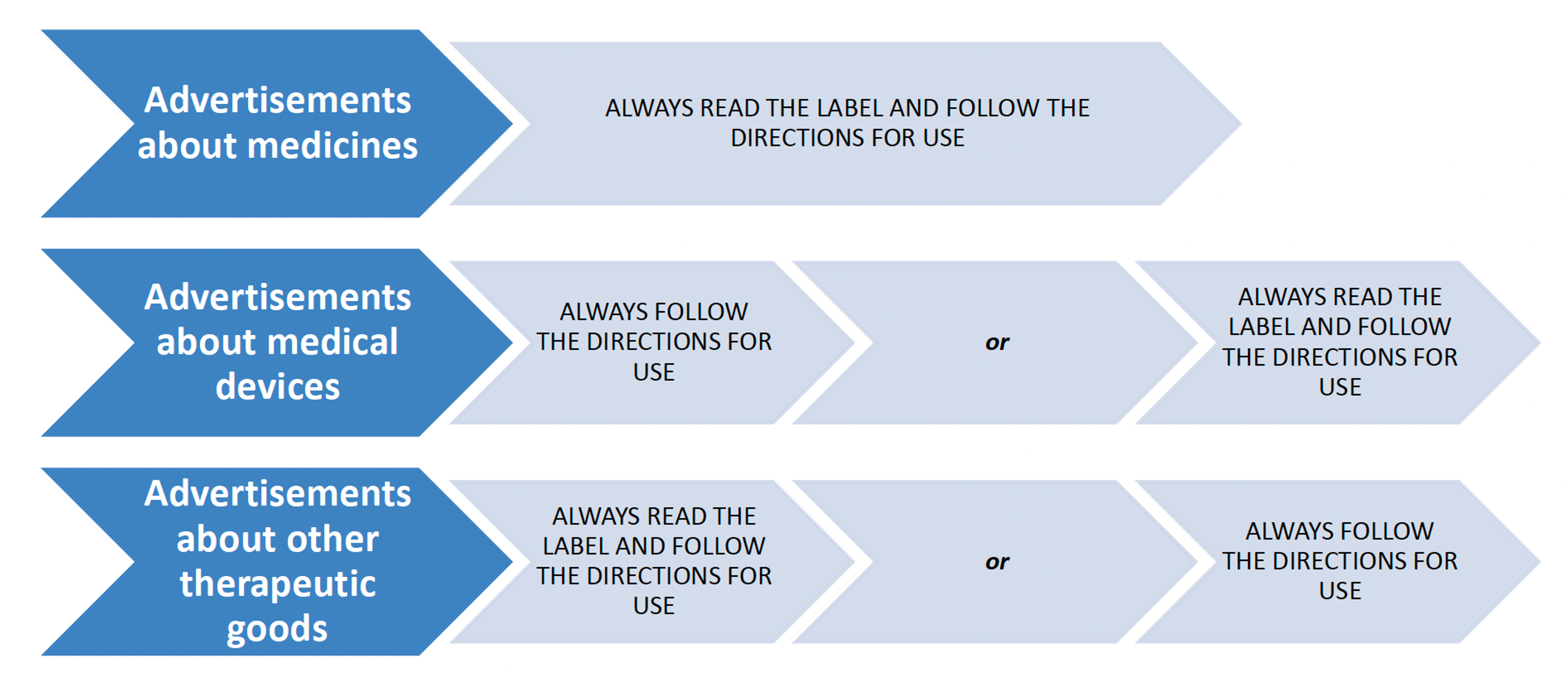
Mandatory statement requirements
Advertisements for therapeutic goods should carry a mandatory statement which is legible and easy to understand. The statements in the 2018 code have been simplified for medicines, medical devices, and other therapeutic goods. The new TGA Code specifies the circumstances in which they are required.
Sampling requirements
Under the new TGA Code, a sample of a therapeutic good amounts to advertising of that therapeutic good, which is prohibited unless identified exemptions apply. Previously, only expressly prohibited “offers” of a sample were clearly non-compliant.
It defines a “sample” as any goods given for free, however does not include a ‘buy one get one free’ offer (provided that the free therapeutic goods are the same as the purchased therapeutic goods).
Despite this, the list of goods that may be offered as samples has been significantly expanded. The list includes COVID-19 related products such as rapid antigen tests, disinfectants, face masks and hand sanitisers as well as blood glucose strips, nicotine replacements therapies, oral hygiene products and female hygiene products.
It will be important to check goodie bags to make sure any therapeutic goods that have been included or will be provided as freebies are included on the approved samples list.
Closing remarks
In addition to these key changes, the new TGA Code:
Please contact the team for further advice on the new Code and look out for more content on this topic by clicking this link.